Comprehensive Cancer Susceptibility Testing (BRCA1, BRCA2 Genetic Testing)
What is BRCA1, BRCA2 Genetic Testing?
Genetic Testing and Lifetime Risk
The analyzed 46 genes are classified into three main groups: high risk, moderate risk, and new risk.
*High-Risk Genes
Individuals with pathogenic mutations in high-risk genes have a risk more than four times higher compared to the general population.
Those with mutations in high-risk genes may have an increased risk of developing cancer or tumors at a young age or developing multiple cancers throughout their lifetime.
High-risk genes include BRCA1, BRCA2 (associated with hereditary breast and ovarian cancer syndrome), CDH1 (hereditary diffuse gastric cancer syndrome), EPCAM, MLH1, MSH2, MSH6, PMS2 (Lynch syndrome), PALB2, PTEN (including PTEN hamartoma tumor syndrome), and TP53 (Li-Fraumeni syndrome).
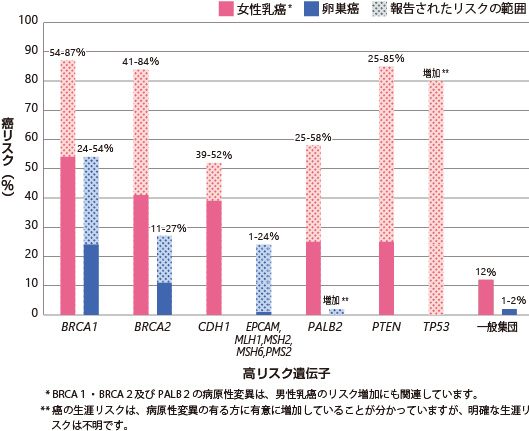
Figure 1: Lifetime Risk of Breast and Ovarian Cancer with Pathogenic Mutations in High-Risk Genes.
*Moderate-Risk Genes
Individuals with mutations in moderate-risk genes have a 2 to 4 times higher risk of developing cancer in their lifetime compared to the general population.
*New-Risk Genes
There are newly identified genes that have been associated with cancer risk, although they may not have been extensively studied yet.
In addition to the genes listed in Table 1, the comprehensive cancer panel also tests the following genes: APC, AXIN2, BMPR1A, CDK4, CDKN2A, MUTYH, POLD1, POLE, SCG5/GREM1, SMAD4, STK11, and VHL.
Table 1: Lifetime Risk of Cancer (Tumors) Associated with Genes Linked to Hereditary Breast and Ovarian Cancer.
|
|
Genes
|
Lifetime Risk of Cancer (Tumors)
|
|
High-Risk Genes
|
BRCA1
|
Female Breast (57-87%), Ovarian (24-54%), Prostate, Male Breast, Pancreas, Fallopian Tube, Primary Peritoneal, Endometrial
|
|
BRCA2
|
Female Breast (41-84%), Prostate (20-34%), Ovarian (11-27%), Pancreas (5-7%), Male Breast (4-7%), Melanoma, Fallopian Tube, Primary Peritoneal, Endometrial
|
|
CDH1
|
Gastric Cancer (40-83%), Female Breast (39-52%), Colon
|
EPCAM,
MLH1,
MSH2,
MSH6,
PMS2
|
Colorectal (11-80%), Endometrial (12-61%), Ovarian (1-24%), Stomach (<1-20%), Urinary Tract (1-10%), Pancreas, Biliary Tract, Small Intestine, Brain, Sebaceous Gland Tumor
(Data on the association of specific cancers with pathogenic variants in MSH6, PMS2, and EPCAM, which represent the tumor spectrum of Lynch syndrome, are limited)
|
|
PALB2
|
Female breasts (25-58%), male breasts, pancreas, ovaries.
|
|
PTEN
|
Female breasts (25-85%), thyroid (3-38%), endometrium (5-28%), colon, kidneys, melanoma, gastrointestinal polyps.
|
|
TP53
|
Female breasts, osteosarcoma and soft tissue, brain, hematologic malignancies, adrenal cortical carcinoma, etc.
Overall risk of cancer: nearly 100% in females, 73% in males.
|
|
Moderate-risk genes
|
ATM
|
Female breasts, colon, pancreas
|
|
BRIP1
|
Ovaries, female breasts
|
|
CHEK2
|
Female breasts, male breasts, colon, prostate, thyroid, endometrium, ovaries
|
|
RAD51C
|
Ovaries, female breasts
|
|
RAD51D
|
Ovaries, female breasts
|
|
Novel risk genes
|
BARD1
|
Female breasts, ovaries
|
|
FANCC
|
Female breasts
|
|
NBN
|
Female breasts, melanoma, non-Hodgkin lymphoma
|
Sample submission
The test is conducted by collecting DNA from the oral mucosa.
To undergo the test, submission of a questionnaire, consent form, and request form is required.
Results
Results will be available in approximately 2-4 weeks.
The test results are classified into the following four categories:
-
Positive (pathogenic mutation present)
-
Suspicion of pathogenic mutation
-
Negative (no pathogenic mutation)
-
Variant of uncertain significance
Medical management based on genetic test results
Medical management for early detection or risk reduction will be recommended based on the results.
Medical management may include enhanced screening, risk-reducing surgeries, and, in some cases, medication for risk reduction.
Recommended screening tests may include MRI, mammogram, ultrasound, endoscopic examination, and biopsy.
Limitations of the test
Even with comprehensive cancer susceptibility testing, there are various types of cancer, and there is still a possibility of developing cancer in the future.
In most cases, it is not believed that the risk of cancer will be significantly higher than that of the general population who received negative test results.
If described as moderate-risk or novel risk genes, the interpretation of the results may be limited.
Even if the result is negative, there may be other genes related to familial tumors or genetic regions that were not detected in the initial test.
Genetic specialists or other healthcare providers may determine the need for further genetic testing.
Benefits of the test
Typically, if the result is positive or suggestive of a pathogenic mutation, most often, relatives (parents, siblings, and children) have a 50% chance of having the same mutation.
While not everyone inheriting pathogenic or potentially pathogenic mutations in these genes will develop cancer or tumors, please note that they have a higher likelihood compared to the general population.
In some cases, specific genes may be associated with autosomal dominant conditions.
Knowledge of a positive result provides valuable information to patients, healthcare providers, and their families, helping to reduce risks and improve early detection.
It may also be appropriate for family members to undergo testing, allowing for a more accurate prediction of the risk of cancer or tumors.
Cost
240,000 yen (excluding tax)
Reservations are required for the test, so please contact our staff.
*Please note that the fees are subject to change without prior notice.