The BRCA gene repairs DNA (deoxyribonucleic acid) that has been damaged and mutated by the external environment, such as ultraviolet light and chemicals, and suppresses cancer.
The BRCA protein contained in the BRCA gene repairs mutations in DNA so that cells remain normal.
BRCA1 and 2 Mutations and Their Risks
When the BRCA gene, which is responsible for repairing DNA mutations, is mutated, the BRCA protein it contains becomes abnormal and the DNA mutation is not repaired.
In such cases, abnormal cells with DNA mutations remain and the probability of cancer development increases.
In most cases, the BRCA mutated gene is inherited from both parents.
Inheritance of a mutated BRCA gene tends to increase the probability of developing breast and ovarian cancer at a younger age than inheritance of a normal (unmutated) BRCA gene.
Breast Cancer Risk
|
Group Type
|
Probability of Occurrence
|
|
Probability of developing breast cancer in the population of the general female population
|
About 12% over the course of a lifetime
|
|
Women who have inherited a harmful BRCA1 mutation
|
Estimated at about 72% by age 80
Approximately 40% chance of developing breast cancer in the other breast within 20 years of diagnosis
|
|
Women who have inherited a harmful BRCA2 mutation
|
Estimated at about 69% by age 80
Approximately 26% chance of developing breast cancer in the other breast within 20 years of diagnosis
|
Ovarian Cancer Risk
About BRCA 1 and 2 Tests
It is important to know whether or not you have a BRCA mutated gene, but it is not necessary to test all people because people with BRCA mutated genes are rare.
The U.S. Preventive Services Task Force (USPSTF) recommends that women with a family history of breast, ovarian, fallopian tube, or peritoneal cancer are more likely to have a BRCA mutated gene and should therefore receive counseling by a medical professional specializing in cancer genetics.
Risk of Testing for BRCA 1 and 2 Mutations
The psychological impact of taking a genetic mutation test and learning about the probability of developing cancer from the results may be felt.
In addition, because it involves not only themselves but also their families and relatives, there is a good chance that the outcome may affect their social and economic thinking and choices, as well as their choice of medical treatment.
It is possible that the results may not be 100%.
For this reason, we strongly recommend that you seek counseling from a medical professional specializing in cancer genetics before undergoing testing.
If a Family Member with a Possible BRCA Mutation Has Developed Cancer
If a family member with a possible BRCA mutation has developed cancer and is still alive, we strongly recommend that they be tested for the gene mutation if they agree to undergo the test.
Family Members Under 18 Years of Age with Possible BRCA Gene Mutation
Genetic testing is not recommended among professionals when a person is under 18 years of age.
This is because the incidence of cancer is extremely low in those under 18 years of age, and there are a variety of possible risks.
About Ashkenazi Jews
Ashkenazi Jews have a higher rate of hereditary breast and ovarian cancer syndrome (HBOC) associated with the BRCA1 and BRCA2 genes and are estimated to have a 10-fold higher risk of developing hereditary breast and ovarian cancer than the general population excluding Ashkenazi Jews.
Possible Criteria for BRCA 1 and 2 Mutations
1.Diagnosed with breast cancer before age 50.
2.Cancer has developed in both breasts.
3.One woman has had multiple cases of breast and ovarian cancer.
4.One woman in the family has had multiple cases of breast and ovarian cancer.
5.A man has developed breast cancer.
6.Two or more primary cancers in the family that are thought to be caused by BRCA1 or BRCA2.
7.Ashkenazi Jew
Test(Sample Submission) and Test Results
Test(Sample Submission)
The test is performed by collecting DNA from the oral mucosa.
To undergo testing, a medical questionnaire, consent form, and request form must be submitted.
Test Results
Results are available in approximately 2-4weeks. Test results fall into four categories
1.Positive (with pathogenic mutation)
2.Suspected pathogenic mutation
3.Negative
4.Mutations of unknown significance
Medical Management based on Genetic Test Results
Depending on your results, you will receive medical management for early detection or risk reduction.
Medical management includes enhanced screening, risk reducing surgery and occasionally risk reducing medicine.
The recommended screening would include MRI, mammogram, ultrasound, endoscopy, and biopsy, etc.
Merits and Limitations of Test
Merits of Test
Usually most positive or likely pathogenic test results, first-degree relatives (parents, siblings, and children) have a 50% chance of having the same variant.
Please remember that for most of these genes, not all people who inherit a pathogenic or likely pathogenic variant will develop cancer and/or tumours, however the chance is increased above that of the general population.
In some cases, certain genes may also be associated with an autosomal recessive condition.
Knowledge of a positive result provides valuable information to patients, healthcare providers, and family members, reduce risk or improve early detection.
Also, testing family members may be appropriate and can allow for more accurate predictions of their cancer and/or tumour risks.
Limitations of Test
Even if you take this comprehensive test, there are lots of different types of cancers and you may still develop cancer in the future.
In most cases, the risk for cancer is not expected to be greater than the general population with negative test results.
Sometimes the result interpretation may be limited, if the gene is described as moderate-risk or newer-risk.
Even with a negative result sometimes there are other genes that can explain the familial cancer, or areas of a gene that were not examined in the initial test.
A genetic specialist or other healthcare provider can determine if further genetic testing is appropriate.
Cost
¥240,000 (¥264,000 including tax)
Price is subject to change without notice.
Please contact our staff for more information.
How to Apply for the Test
An appointment is necessary for the test, so please ask the staff.
(TEL)070-1820-0909
(mail)
english_help@oakclinic-group.com
Genes and Lifetime Risk
The 46 genes analyzed by this testing fall into three groups: high risk, moderate risk, and new risk.
・High Risk Genes
Those with pathological mutations in high-risk genes have disease risks related to those genes at least four times greater than the general populations.
Mutations on in high-risk genes lead to increased risk of developing cancer or tumors at an early age or developing multiple cancers throughout life.
High-risk genes include BRCA1, BRCA2 (BRCA-related breast or ovarian cancer syndrome); CDH1 (hereditary diffuse gastric cancer syndrome); EPCAM, MLH1, MSH2, MSH6, PMS2 (Lynch syndrome); PALB2; PTEN (Cauden syndrome) Includes PTEN Hamatoma Tumor Syndrome); TP53 (Li-Fraumeni Syndrome).
Figure 1: Lifetime risk of breast and ovarian cancer in the presence of pathogenic mutations in
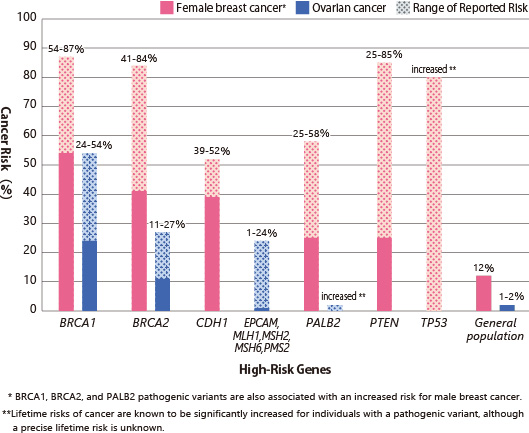
Source:
Latest information on cancer treatment and cancer | Reference "BRCA1, BRCA2 genes: cancer risk and genetic testing"
・Moderate Risk Genes
Mutations in moderate-risk genes lead to the person being two to four times more likely to develop cancer in their lifetime than in the general population.
・New Risk Genes
These are genes newly associated with the risk of cancer, whose exact role and results are not yet well-understood or fully studied.
In addition to Table 1, the Comprehensive Cancer Panel also tests the following genes: APC, AXIN2, BMPR1A, CDK4, CDKN2A, MUTYH, POLD1, POLE, SCG5 / GREM1, SMAD4, STK11, VHL.
Table 1: Lifetime risk of cancer (tumor) linked to genes associated with hereditary breast and ovarian cancer
|
|
Gene
|
Lifetime Cancer and/or Tumor Risks
|
|
High Risk Genes
|
BRCA1
|
Female breast (57-87%), Ovarian (24-54%), Prostate, Male breast, Pancreatic, Fallopian tube, Primary peritoneal, Endometrial
|
|
BRCA2
|
Female breast (41-84%), Prostate (20-34%), Ovarian (11-27%), Pancreatic, Male breast, Melanoma, Fallopian tube, Primary peritoneal, Endometrial
|
|
CDH1
|
Gastric cancer (40-83%), Female breast (39-52%), Colon
|
|
EPCAM,
MLH1,
MSH2,
MSH6,
PMS2
|
Colorectal (11-80%), Endometrial (12-61%), Ovarian (1-24%), Gastric (<1-20%), Urinary tract (1-10%), Pancreatic, Biliary tract, Small bowel, Brain, Sebaceous tumors
Tumor spectrum is representative of Lynch syndrome; data are limited with regard to the association of certain cancers with pathogenic variants in MSH6, PMS2 and EPCAM
|
|
PALB2
|
Female breast (25-58%), Male breast, Pancreatic, Ovarian
|
|
PTEN
|
Thyroid (3-38%), Endometrial (5-28%), Colon, Renal, Melanoma, Gastrointestinal polyps
|
|
TP53
|
Female breast, Sarcoma-bone and soft tissue, Brain, Hematologic malignancies, Adrenocortical carcinoma, among others.
Overall risk for cancer: nearly 100% in females, 73% in males.
|
|
Moderate-Risk Genes
|
ATM
|
Female breast, Colon, Pancreatic
|
|
BRIP1
|
Ovarian, Female breast
|
|
CHEK2
|
Female breast, Male breast, Colon, Prostate, Thyroid, Endometrial, Ovarian
|
|
RAD51C
|
Ovarian, Female breast
|
|
RAD51D
|
Ovarian, Female breast
|
|
Newer-Risk Genes
|
BARD1
|
Female breast, Ovarian
|
|
FANCC
|
Female breast
|
|
NBN
|
Female breast, Melanoma, Non-Hodgkin lymphoma
|